For thousands of women, transvaginal mesh has caused more problems than it has solved. Women with TVM have reported infections, urinary problems, pain during sexual intercourse, scarring, bladder perforation, recurrence of pelvic organ prolapse or incontinence, and other problems. If you have a transvaginal mesh implant and now suffer unusual pain or other symptoms, you need to take action quickly.
Depuy Pinnacle Hip Cases Currently Being Tried to Jury in Texas
There is a very important Depuy Pinnacle hip case being tried in Dallas, Texas as I write this.
The federal court system has consolidated many of the Depuy Pinnacle lawsuits into one multidistrict litigation in the North District of Texas (3:11-md-02244) presided over by federal judge Ed Kinkeade.
Bellwether Cases
Back in August, Judge Kinkeade selected certain cases as “bellwether selections” and ordered the parties to organize those cases for jury trials. Bellwether cases are representative cases which have broad characteristics in common with many of the remaining cases.
So Judge Kinkeade ordered that five separate cases would be consolidated into one (very large) jury trial, to start January 8. Those five plaintiffs are:
- Aoki
- Christopher
- Greer
- Klusmann
- Peterson
One jury will hear all the evidence in these cases, but the judge will allow the jury to consider liability in each case, and to award separate damages in each case. Based on court filings, all five plaintiffs are from Texas, and each case has many similarities, thus making them amenable to trying together. In the language of the law, these five cases have “common issues of law and fact.” Opening arguments began January 11. The case is supposed to last three months.
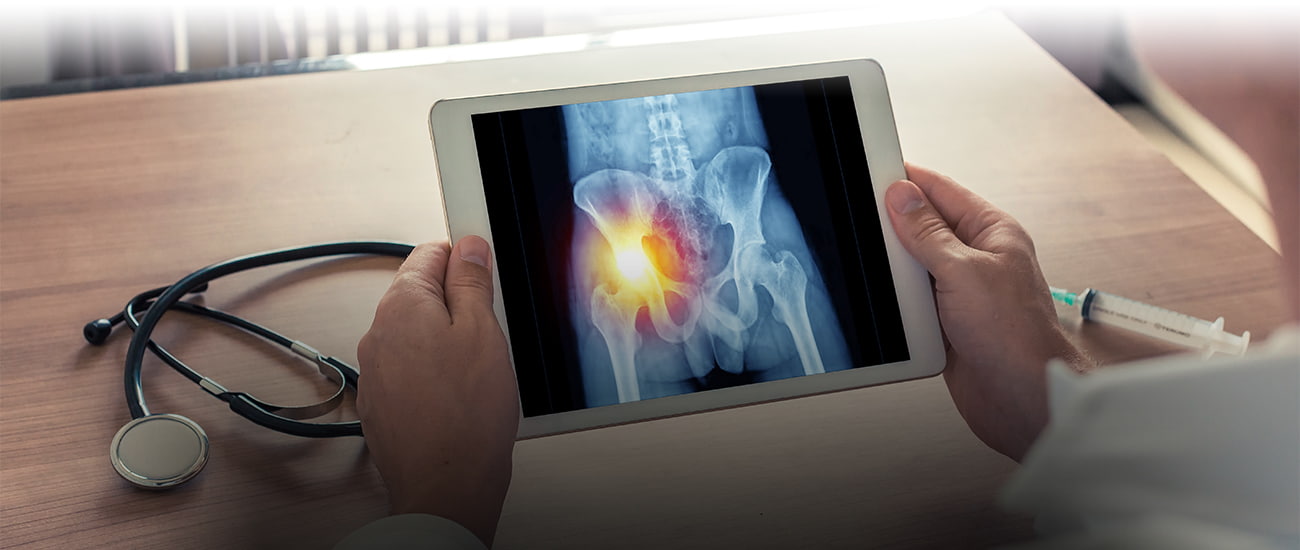
Failed Artificial Hips: What Cases Are Still Active in 2016?
Artificial hips have been failing for years now. The effort in the last two decades to build and market a metal-on-metal hip that might last the patient’s lifetime has not been a success. The manufacturers were too quick to move the new metal hips to market, and using the flawed 510(k) fast-track process, they did not test the devices sufficiently to understand the risks. As a result, thousands of people suffered through hip replacement surgery failure and had to undergo revision surgery, just months or a few years after the original surgery. Because of these hip failures, thousands of lawsuits have been filed. The litigation continues for many people, and will continue for years. Still, because most of the failed medical hip products were recalled or taken off the market years ago, the litigation is plainly winding down with several of these artificial hip devices. So what’s still going on? And what does this mean for you?
Transvaginal Mesh Reclassified by FDA as Highest Risk Device
Mark this down in the “It’s About Time” column.
On January 4, 2016, the Food and Drug Administration (FDA) finally made the move to reclassify transvaginal mesh medical products from Class II to Class III.
Surgical mesh is a medical product that has been used for more than sixty years and is intended to provide “additional support when repairing weakened or damaged tissue.” FDA News Release. It is typically a plastic lattice-type mesh that is surgically implanted around weakened, loose human tissue (such as a vaginal wall). While surgical mesh can be used to treat different kinds of problems, such as hernia, the mesh that has caused much of the current suffering and most of the lawsuits involves transvaginal mesh (TVM). Pelvic organ prolapse (POP), where organs fall from their normal position and press against the vaginal walls, is one of the conditions treated by TVM.
Recent Transvaginal Mesh Trials: Two Losses, One Win for Injured Women (Part 4)
We’ve been looking at recent trial results in transvaginal mesh cases. In this (fourth) post we review two verdicts from October 2015, where juries concluded that the mesh manufacturers were not negligent. Thus, these injured women received no money for the failure of the transvaginal mesh. In a third case, decided on December 21, 2015, the jury awarded $12,500,000.00 to an injured woman. I will take a look at all three cases:
Cavness v. Kowalczyk et al. (Texas District Court, Dallas)
Product: Gynecare Prosima Pelvic Floor Repair System
Key Product Defendants: Ethicon, Inc. and Johnson & Johnson.
Jury Award: zero.
Date of Jury Verdict: October 5, 2015
Key Takeaway: A big win for Ethicon and Johnson & Johnson, defendants in the case.
Transvaginal Mesh: One Huge Verdict and One Key Court Ruling from 2015 (Part 3)
In this post, we continue our review of transvaginal mesh cases. We look at one (remarkable) jury verdict and one partial summary judgment decision in favor of the defendant from 2015. Many other cases are moving to trial. Going forward, I will keep you updated on jury verdicts and other key court decisions as they happen.
Barba v. Boston Scientific Corp. (Delaware Superior Court)
Products: Pinnacle Pelvic Floor Repair Kit Transvaginal Mesh and Advantage Fit Mid-Urethral Sling System
Jury Award: $100,000,000.00.
Date of Jury Verdict: May 28, 2015
Key Takeaway: A huge verdict for the plaintiff, Deborah Barba. This case was remarkable for the very high money award: one hundred million dollars, to a single woman.
Transvaginal Mesh Cases: Two Jury Verdicts and One Appeal (Part 2)
Transvaginal mesh lawsuits are finally getting their day in court. This is a good thing for the thousands of women who were injured, some severely, by the failure of the transvaginal mesh products sold by Ethicon (Johnson & Johnson), Boston Scientific, and other companies. In Part 1, we looked at three TVM jury verdicts from 2013 and 2014. In this post, we look at two jury verdicts and an appeal decision from November 2014.
Many other cases are moving to trial. In Part 3, I will discuss several jury verdicts from 2015.
Transvaginal Mesh Lawsuits: Key Jury Verdicts (Part 1)
Transvaginal mesh (TVM) is a plastic mesh product that is surgically implanted in women to repair or support weakened vaginal walls and other compromised tissue. Many women suffer from a condition called pelvic organ prolapse (POP), where an organ like the bladder prolapses from its normal position in the body and presses against the walls of the vagina. One of the main causes of this condition is childbirth. The FDA approved TVM for treatment of women with POP and other conditions like stress urinary incontinence more than a decade ago.
Sadly, TVM has caused terrible problems for thousands of women. Not only after transvaginal mesh was marketed and sold, women began complaining of different, often more serious problems. Complications include erosion of the vaginal wall, infections, urinary problems, pain during sexual intercourse, scarring, bowel or bladder perforation, and recurrence of pelvic organ prolapse or incontinence. Women have been forced to undergo surgeries to attempt to repair the damage done by transvaginal mesh. In some cases injured women have endured multiple surgeries.
The lawsuits followed. Fortunately for these (often severely) injured women, these cases are finally getting to trial and to jury verdicts. The good news is that some plaintiffs have received millions from juries for their injuries. But the TVM manufacturers have also scored victories.
Transvaginal Mesh: What Is It and Why So Many Lawsuits?
You have probably seen news reports (or at least the flood of TV commercials from law firms) about the suffering caused by the failure of transvaginal mesh (TVM) products. This is a unique medical device failure “story” because of the large number of women who have been affected. It is estimated that transvaginal mesh is implanted in more than 200,000 women each year in the United States. And by early 2015 well over 70,000 lawsuits have been filed against transvaginal mesh manufacturers because of injuries caused by TVM. More lawsuits are being filed each week.
But let’s back up.
Surgical mesh is a medical product that “is used to provide additional support when repairing weakened or damaged tissue.” FDA News Release April 29, 2014. It is a plastic lattice-type mesh that is surgically implanted around weakened, loose, sagging, or otherwise compromised human tissue (such as a vaginal wall). While surgical mesh can be used to treat different kinds of problems, such as hernia, the mesh that has caused much of the current suffering and most of the lawsuits involves transvaginal mesh.
Three Key Verdicts in 2015 Risperdal Jury Trials
I have written on this site about the horrific side effects that some young men have suffered as a result of taking the antipsychotic drug Risperdal. Check out my recent post on the subject for further information.
Risperdal was developed by Janssen Pharmaceuticals (a company owned by Johnson & Johnson) to treat symptoms of schizophrenia and bipolar disorder. Unfortunately, Risperdal has caused terrible side effects, including gynecomastia, which is the growth of female breasts on boys and young men. As you can imagine, once this condition developed, and then developed again and again in many young men, it became clear that something was very wrong.
The lawsuits followed, over 5,000 so far, and more are being filed each week.
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog