 Exactech Inc. has been in business since 1985. The company focuses on developing products for joint replacements, including hip, knee, and ankle replacements.
Exactech Inc. has been in business since 1985. The company focuses on developing products for joint replacements, including hip, knee, and ankle replacements.
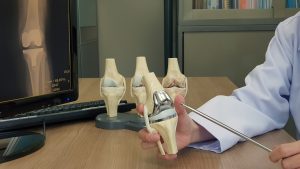
Exactech’s components for joint replacement have been useful, and may have performed well over the years. However, in June of 2021, Exactech Inc. had to issue a series of recalls for several different plastic liner implants that fit within the normal artificial hip, knee, and ankle replacements because they caused a great deal of problems for patients.
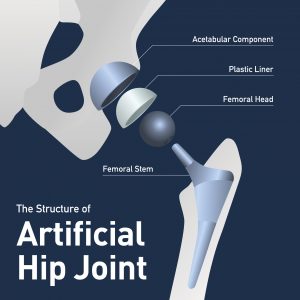
In this podcast, attorney Clay Hodges gives an overview of the series of recalls that Exactech has issued for its polyethylene liners for hip, knee, and ankle joint replacement surgeries that will lead to litigation. Between 2008 and 2021, more than 89,000 Exactech Connexion GXL Hip Liners from Exactech got implanted worldwide, and since 2004, Exactech has sold 140,732 liner tibial base plates for knee replacements.
Exactech is taking this problem seriously. So if you have had a hip, knee, or ankle replacement done in the last 15 years, stay tuned to find out how to know if you have one of Exactech’s recalled products implanted in your body and what you can do if you have one.
Show highlights:
- What have studies shown about the polyethylene used to manufacture the plastic liners for artificial hip replacement surgery?
- Why is the polyethylene liner so important in artificial hip surgery?
- Some problems that the breaking down of Exactech’s hip plastic liners caused.
- Check out your surgical records to see which surgical products were placed in your body if you have been experiencing problems with an artificial hip replacement done in the last 15 years. You might qualify for litigation or need to have revision surgery.
- Check on the FDA website to see if you have a recalled product implanted in your body.
- From 2008 to April of 2021, more than 89,000 Connexion GXL Hip Liners from Exactech were implanted in people worldwide.
- The second group of recalls is related to Exactech’s plastic liners for knee and ankle replacement products.
- The liners for knee and hip replacements were made from ultra-high molecular weight polyethylene (UHMWP), which has to be packaged in special oxygen-resistant vacuum bags.
- One of the key problems with the knee replacement liners was caused by this defective packaging.
- The potential problems the recalled models of liner tibial base plates for knee replacements, manufactured and sold by Exactech, could cause.
- The ankle product is a Vantage fixed-bearing liner. There might be only about 1500 of those products out there.
Links and resources:
If you think you may have a case against Exactech, call Clay at 919-546-8788.
Check out Clay Hodges’s website
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog